by Jonathan G Pattrick, William Block & Beverley J Glover –
Abstract
Grooming is a honey bee behavior that has the potential to minimize and manage the detrimental effects of Varroa destructor. Here we tested the efficacy of the Bee Gym™, a device hypothesized to increase honey bee auto-grooming and increase mite removal from colonies. Natural mite fall from 20 colonies was counted for 14 days, after which half the colonies were fitted with a Bee Gym and half with a control object. Mite fall and the proportion of damaged mites were then recorded for another 14 days. Total mite fall was generally higher over the second 14 days, but this increase was not significantly higher for the Bee Gym colonies than for the control colonies. There was also no difference in the proportion of damaged mites between the two treatments. Mite fall and damage to mites may be influenced by other factors, and this is discussed; however, given that we found no effect of the Bee Gym, we conclude that there is no evidence from this study of its efficacy as a management strategy for V. destructor.
Introduction
Varroa destructor Anderson and Trueman 2000, is a major pest of Apis mellifera L. 1758, and is present in the majority of honey bee colonies worldwide (Rosenkranz, Aumeier, & Ziegelmann, 2010Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]). V. destructor reproduce in honey bee brood cells (Boecking & Genersch, 2008Boecking, O., & Genersch, E. (2008). Varroosis—The ongoing crisis in bee keeping. Journal Fur Verbraucherschutz Und Lebensmittelsicherheit, 3, 221–228. doi:10.1007/s00003-008-0331-y[CrossRef], [Web of Science ®] [Google Scholar]), and directly harm the bees by attaching to and feeding from the haemolymph of the brood and the adults (Bowen-Walker & Gunn, 2001Bowen-Walker, P.L., & Gunn, A. (2001). The effect of the ectoparasitic mite, Varroa destructor on adult worker honey bee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomologia Experimentalis et Applicata, 101, 207–217. doi:10.1046/j.1570-7458.2001.00905.x[CrossRef], [Web of Science ®] [Google Scholar]). They also act as vectors for several bee viruses (e.g., Bowen-Walker, Martin, & Gunn, 1999Bowen-Walker, P.L., Martin, S.J., & Gunn, A. (1999). The transmission of deformed wing virus between honey bees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoniOud. Journal of Invertebrate Pathology,73, 101–106. doi:10.1006/jipa.1998.4807[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]). These detrimental effects may act synergistically with other stressors and, without management, the majority of V. destructor-infested colonies will collapse (Guzmán-Novoa et al., 2010Guzmán-Novoa, E., Eccles, L., Calvete, Y., McGowan, J., Kelly, P., & Correa-Benítez, A. (2010). Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie, 41, 443–450. doi:10.1051/apido/2009076[CrossRef], [Web of Science ®] [Google Scholar]; Le Conte, Ellis, & Ritter, 2010Le Conte, Y., Ellis, M., & Ritter, W.(2010). Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie, 41, 353–363. doi:10.1051/apido/2010017[CrossRef], [Web of Science ®] [Google Scholar]; Rinderer et al., 2001Rinderer, T.E., De Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V., … Harris, J.W.(2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie, 32, 381–394. doi:10.1051/apido:2001138[CrossRef], [Web of Science ®] [Google Scholar]).
There are several strategies available to beekeepers for managing V. destructor numbers (e.g., Calderone, 2005Calderone, N. (2005). Evaluation of drone brood removal for management of Varroa destructor(Acari: Varroidae) in colonies of Apis mellifera (Hymenoptera: Apidae) in the northeastern United States. Journal of Economic Entomology, 98, 645–650. doi:10.1603/0022-0493-98.3.645[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Rademacher & Harz, 2006Rademacher, E., & Harz, M. (2006). Oxalic acid for the control of varroosis in honey bee colonies—A review. Apidologie, 37, 98–120. doi:10.1051/apido:2005063[CrossRef], [Web of Science ®] [Google Scholar]; and see Rosenkranz et al., 2010Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®] [Google Scholar] for a review), although each has its limitations (e.g., Higes, Meana, Suárez, & Llorente, 1999Higes, M., Meana, A., Suárez, M., & Llorente, J. (1999). Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoniOud. Apidologie, 30, 289–292. doi:10.1051/apido:19990404[CrossRef], [Web of Science ®] [Google Scholar]; Lodesani, Colombo, & Spreafico, 1995Lodesani, M., Colombo, M., & Spreafico, M. (1995). Ineffectiveness of Apistan® treatment against the mite Varroa jacobsoni Oud in several districts of Lombardy (Italy). Apidologie, 26, 67–72. doi:10.1051/apido:19950109[CrossRef], [Web of Science ®] [Google Scholar]; Martin, 2004Martin, S.J. (2004). Acaricide (pyrethroid) resistance in Varroa destructor. Bee World, 85, 67–69. doi:10.1080/0005772X.2004.11099632[Taylor & Francis Online], [Web of Science ®] [Google Scholar]). The effort and money required to treat V. destructor has likely been a factor in making beekeeping less attractive (Potts et al., 2010Potts, S.G., Roberts, S.P.M., Dean, R., Marris, G., Brown, M., Jones, R., & Settele, J. (2010). Declines of managed honey bees and beekeepers in Europe. Journal of Apicultural Research,49, 15–22. doi:10.3896/IBRA.1.49.1.02[Taylor & Francis Online], [Web of Science ®] [Google Scholar]; Rosenkranz et al., 2010Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]) and, given the current limits in treatment methods, there is a need for new solutions for managing infestations.
Some A. mellifera strains are resistant to the mites (Martin & Medina, 2004Martin, S.J., & Medina, L.M. (2004). Africanized honey bees have unique tolerance to Varroa mites. Trends in Parasitology, 20, 112–114. doi:10.1016/j.pt.2004.01.001[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Rinderer et al., 2001Rinderer, T.E., De Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V., … Harris, J.W.(2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie, 32, 381–394. doi:10.1051/apido:2001138[CrossRef], [Web of Science ®] [Google Scholar]) giving hope for alternative approaches to managing V. destructor. Two behavioral traits have been identified that may contribute to the resistance: Varroa-sensitive hygiene and grooming (Boecking & Spivak, 1999Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30, 141–158. doi:10.1051/apido:19990205[CrossRef], [Web of Science ®] [Google Scholar]; Rinderer, Harris, Hunt, & de Guzman, 2010Rinderer, T.E., Harris, J.W., Hunt, G.J., & de Guzman, L.I. (2010). Breeding for resistance to Varroa destructor in North America. Apidologie, 41, 409–424. doi:10.1051/apido/2010015[CrossRef], [Web of Science ®] [Google Scholar]). Varroa-sensitive hygiene is the ability of bees to remove infested brood (Evans & Spivak, 2010Evans, J., & Spivak, M. (2010). Socialized medicine: Individual and communal disease barriers in honey bees. Journal of Invertebrate Pathology,103, S62–72. doi:10.1016/j.jip.2009.06.019[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Harris, 2007Harris, J.W. (2007). Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged ≤ five days post capping. Journal of Apicultural Research, 46, 134–139. doi:10.3896/IBRA.1.46.3.02[Taylor & Francis Online], [Web of Science ®] [Google Scholar]), and there has been some success breeding honey bee strains with this trait that have increased resistance to V. destructor (Rinderer et al., 2010Rinderer, T.E., Harris, J.W., Hunt, G.J., & de Guzman, L.I. (2010). Breeding for resistance to Varroa destructor in North America. Apidologie, 41, 409–424. doi:10.1051/apido/2010015[CrossRef], [Web of Science ®] [Google Scholar]). In contrast, evidence that grooming leads to increased resistance is poor for A. mellifera, and the breeding of high-grooming, mite-resistant lines of bees has not yet been successful (Rinderer, De Guzman, & Frake, 2013Rinderer, T.E., De Guzman, L.I., & Frake, A.M. (2013). Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology, 106, 566–575. doi:10.1603/EC12427[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]).
Despite this, many studies have demonstrated that A. mellifera are able to remove mites through grooming, even though they are not always very effective at doing so. Evidence for this has been shown both at the colony level (Arechavaleta-Velasco & Guzmán-Novoa, 2001Arechavaleta-Velasco, M., & Guzmán-Novoa, E. (2001). Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie, 32, 157–174. doi:10.1051/apido:2001121[CrossRef], [Web of Science ®] [Google Scholar]; Guzmán-Novoa, Emsen, Unger, Espinosa-Montaño, & Petukhova, 2012Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Mondragón, Spivak, & Vandame, 2005Mondragón, L., Spivak, M., & Vandame, R. (2005). A multifactorial study of the resistance of honey bees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie, 36, 345–358. doi:10.1051/apido:2005022[CrossRef], [Web of Science ®] [Google Scholar]; Rinderer et al., 2001Rinderer, T.E., De Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V., … Harris, J.W.(2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie, 32, 381–394. doi:10.1051/apido:2001138[CrossRef], [Web of Science ®] [Google Scholar]) and through assays inoculating individual bees with mites (Aumeier, 2001Aumeier, P. (2001). Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie,32, 81–90. doi:10.1051/apido:2001113[CrossRef], [Web of Science ®] [Google Scholar]; Büchler, Drescher, & Tornier, 1992Büchler, R., Drescher, W., & Tornier, I.(1992). Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsataand its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Experimental and Applied Acarology, 16, 313–319. doi:10.1007/BF01218573[CrossRef], [Web of Science ®] [Google Scholar]; Fries, Huazhen, Wei, & Jin, 1996Fries, I., Huazhen, W., Wei, S., & Jin, C.S. (1996). Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie, 27, 3–11. doi:10.1051/apido:19960101[CrossRef], [Web of Science ®] [Google Scholar]; Guzmán-Novoa et al., 2012Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Peng, Fang, Xu, & Ge, 1987Peng, Y., Fang, Y., Xu, S., & Ge, L.(1987). The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. Journal of Invertebrate Pathology, 49, 54–60. doi:10.1016/0022-2011(87)90125-X[CrossRef], [Web of Science ®] [Google Scholar]). These are reviewed in detail by Rinderer et al. (2013Rinderer, T.E., De Guzman, L.I., & Frake, A.M. (2013). Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology, 106, 566–575. doi:10.1603/EC12427[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]), and also see Pritchard (2016Pritchard, D.J. (2016). Grooming by honey bees as a component of varroa resistant behavior. Journal of Apicultural Research, 55, 38–48. doi:10.1080/00218839.2016.1196016[Taylor & Francis Online], [Web of Science ®] [Google Scholar]).
Grooming behavior can be split into auto-grooming, where a bee grooms itself, and allo-grooming, in which a bee is groomed by others, often initiated through a recruitment dance (Evans & Spivak, 2010Evans, J., & Spivak, M. (2010). Socialized medicine: Individual and communal disease barriers in honey bees. Journal of Invertebrate Pathology,103, S62–72. doi:10.1016/j.jip.2009.06.019[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Land & Seeley, 2004Land, B.B., & Seeley, T.D. (2004). The grooming invitation dance of the honey bee. Ethology, 110, 1–10. doi:10.1046/j.1439-0310.2003.00947.x[CrossRef], [Web of Science ®] [Google Scholar]). In A. mellifera, auto-grooming seems to be the most frequently observed behavior in response to mites (Aumeier, 2001Aumeier, P. (2001). Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie,32, 81–90. doi:10.1051/apido:2001113[CrossRef], [Web of Science ®] [Google Scholar]; Fries et al., 1996Fries, I., Huazhen, W., Wei, S., & Jin, C.S. (1996). Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie, 27, 3–11. doi:10.1051/apido:19960101[CrossRef], [Web of Science ®] [Google Scholar]). This typically involves bees wiping their body with their legs, but can include the bees grasping mites with their mandibles (Boecking & Spivak, 1999Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30, 141–158. doi:10.1051/apido:19990205[CrossRef], [Web of Science ®] [Google Scholar]; Guzmán-Novoa et al., 2012Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]).
More substantial evidence that grooming is potentially important for mite resistance comes from Apis cerana Fabricus 1793, the natural host of V. destructor. A. cerana are in a balanced host-parasite relationship with the mites and, although other factors may play a part, grooming is believed to contribute to this (Boecking & Spivak, 1999Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30, 141–158. doi:10.1051/apido:19990205[CrossRef], [Web of Science ®] [Google Scholar]; Rosenkranz et al., 2010Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]). Notably, the grooming behavior of A. cerana in response to the mites is stronger and more effective than in A. mellifera (Boecking & Spivak, 1999Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30, 141–158. doi:10.1051/apido:19990205[CrossRef], [Web of Science ®] [Google Scholar]; Büchler et al., 1992Büchler, R., Drescher, W., & Tornier, I.(1992). Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsataand its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Experimental and Applied Acarology, 16, 313–319. doi:10.1007/BF01218573[CrossRef], [Web of Science ®] [Google Scholar]; Fries et al., 1996Fries, I., Huazhen, W., Wei, S., & Jin, C.S. (1996). Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie, 27, 3–11. doi:10.1051/apido:19960101[CrossRef], [Web of Science ®] [Google Scholar]; Peng et al., 1987Peng, Y., Fang, Y., Xu, S., & Ge, L.(1987). The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. Journal of Invertebrate Pathology, 49, 54–60. doi:10.1016/0022-2011(87)90125-X[CrossRef], [Web of Science ®] [Google Scholar]; Rosenkranz et al., 2010Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]). This suggests that methods to enhance grooming in A. mellifera, either through selecting high-grooming lines or through improving the effectiveness of the behavior in other ways, could increase mite removal and colony resistance.
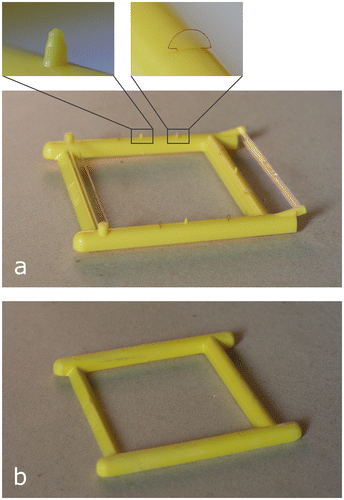
The Bee Gym™ (http://www.beegym.co.uk/) is a device proposed to effect mite removal from bee colonies by increasing auto-grooming behavior. It consists of a 110 × 117 mm plastic frame with a range of rough protrusions such as spikes and semi-rigid flaps (Figure 1(a)). It is hypothesized to work by functioning as a “scratching post”, allowing adult workers to actively groom themselves against the protrusions, increasing mite removal from the colony. It is designed to be placed in hives throughout the year, either directly underneath or above the brood frames (Stuart Roweth, Personal Communication, 2014). As a potential management strategy that is non-chemical, cheap, and easy to implement, the Bee Gym could be a valuable addition to the catalog of methods for managing V. destructor.
Figure 1. (a) The Bee Gym, with close-ups of two of the nine grooming protrusions. These vary in stiffness. (b) The Control Frame, a Bee Gym with all grooming protrusions removed or sanded to be smooth.
Here we tested the function of the Bee Gym in an apiary trial by recording mite fall from honey bee colonies. If the Bee Gym is effective then, following its introduction to a hive, mite fall should increase above the level expected from normal mite population growth. Mite fall was recorded in 10 hives over two periods of 14 days, before and after Bee Gym treatment. As a control, mite fall was recorded for the same duration in a further 10 hives with no treatment. As a second measure to determine the Bee Gym’s efficacy, the proportion of damaged mites was recorded over the second 14 days.
Materials and methods
Hives and study sites
The trial was completed with 20 honey bee colonies, with a range of V. destructor infestation levels. All colonies were in National hives, and were spread across 4 apiaries: Impington (IM; 10 hives), King’s College (KC; 2 hives), Trinity College (TC; 4 hives) and Cambridge University Botanic Garden (BG; 4 hives). These were all within a 6 km radius of the centre of Cambridge, UK, and thus experienced similar environmental conditions throughout the trial.
Mite counting
All hives were fitted with open mesh floors. The number of mites falling out of the hive was recorded using sticky drop/bottom boards placed beneath the mesh floor at the bottom of the hives (Dietemann, Nazzi, & Martin, 2013Dietemann, V., Nazzi, F., & Martin, S.(2013). Standard methods for varroa research. In V. Dietemann, J.D. Ellis, & P. Neumann (Eds.), The COLOSS BEEBOOK, Volume II: Standard methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research, 52. doi:10.3896/IBRA.1.52.1.09[Taylor & Francis Online] [Google Scholar]). These were coated with a film of petroleum jelly to prevent mites escaping. The number of mites on a board was recorded with the aid of a counting grid. After counting, the drop boards were scraped clean and recoated with petroleum jelly before reinsertion into hives. Mite numbers were counted on average every 2 days, although, as it was rarely possible to count mite fall from all hives on a single day, hives sometimes had 1 or 3 days between counts.
Mite fall was counted for 14 days with no treatment (7 counts per hive). The drop boards were then removed, and, on day 15, the hives received either a Bee Gym (10 hives), or a control frame (10 hives). These were placed onto the mesh floor at the bottom of the hive. The control frame was the same size and material as the Bee Gym but with all rough edges and protrusions removed or sanded down (Figure 1(b)). The hives were given a day to settle following disturbance, with drop boards replaced on day 16. Mite numbers were then recorded for the following 14 days (7 counts per hive). For the second 14 days, counted mites were classified as undamaged or damaged with the aid of a 10 × hand lens. Mites were classed as damaged if they had missing legs or mouthparts or if there were sections of the dorsal shield (idiosoma) or ventral shield missing. Mites with a dented idiosoma were not included as damaged as this has been shown to be ontological (Davis, 2009Davis, A. (2009). Regular dorsal dimples on Varroa destructor—Damage symptoms or developmental origin. Apidologie, 40, 151–162. doi:10.1051/apido/2009001[CrossRef], [Web of Science ®] [Google Scholar]). All living mites were included in the undamaged category. Mite counting started on 30 June and finished on 30 July 2014.
Randomization process and exclusion criteria
In addition to the 20 colonies that completed the trial, a further 11 colonies initially present were removed. Five colonies were removed during the first two weeks. Four colonies had a very low mite infestation and one underwent a colony merger.
The 26 colonies that entered the second two weeks of the trial were assigned the treatment or control randomly, with the following constraints: (1) The number of treatment and control hives was constrained to be equal within apiaries with an even number of hives (IM and BG) and equal across the combined hives from the apiaries with odd numbers of hives (TC and KC) (ensuring within these apiaries that the disparity between control/treatment hives was not greater than one). (2) The treatment was randomised separately for four hives at IM that had low mite fall (again with equal numbers assigned control/treatment). The counting was performed blind with respect to which hives had the Bee Gyms or control frames; the person recording mites did not know the treatment assignments of each hive.
Brood presence/absence was recorded for all colonies during the trial, as this can have significant effects on mite fall (Branco, Kidd, & Pickard, 2006Branco, M., Kidd, N., & Pickard, R.(2006). A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie, 37, 452–461. doi:10.1051/apido:2006010[CrossRef], [Web of Science ®] [Google Scholar]; Lobb & Martin, 1997Lobb, N., & Martin, S. (1997). Mortality of Varroa jacobsoni Oudemans during or soon after the emergence of worker and drone honey bees Apis mellifera L. Apidologie, 28, 367–374. doi:10.1051/apido:19970604[CrossRef], [Web of Science ®] [Google Scholar]). As six colonies had brood absent for a portion of the trial, these were removed from the analyzes, leaving the final 20 colonies that completed the trial.
Statistical analysis
Mite fall was summed for each colony for the 14 days pre-treatment and 14 days with-treatment and adjusted for the actual number of hours each drop board was in place, giving a mean fall per 14 days per colony. The data were analyzed considering both absolute and proportional change in mite fall rate. A two-sample Wilcoxon test was used to test for a difference in the proportional change in mite fall between the Bee Gym and control colonies from the first 14 days to the second 14 days. To investigate absolute changes in mite fall, the data were modeled using a linear regression with mite fall after as response and mite fall before and treatment as predictors. Fall after and before were log transformed using natural logarithms to account for non-constant variance of residuals. To test whether there was a higher proportion of damaged mites in the mite fall from the Bee Gym colonies than the control colonies for the second 14 days, the proportion of damaged mites was modeled using a generalized linear model with a binomial error structure. For two colonies which had a very high daily fall, mites were not split into the two categories due to time restrictions of the recording schedule. This left 18 colonies which were analyzed for damage to mites. All statistics were carried out using R version 3.1.3 (R Core Team, 2015R Core Team. (2015). R: A language and environment for statistical computing (Version 3.1.3). Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]).
Results
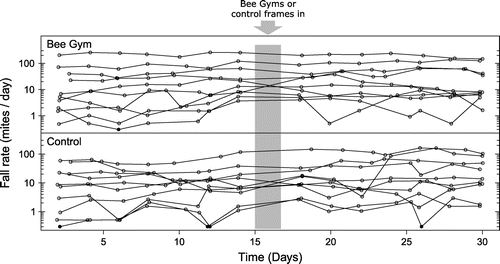
For the 20 analyzed colonies, 18,566 mites were counted over the 28 days. Mite fall varied considerably between colonies. The colony with the highest fall had a mean daily fall rate of 212 mites per day, and seven further colonies had daily fall rates higher than 30 mites per day. In contrast, nine colonies had a mean daily fall rate lower than 10 mites per day. The remaining three colonies had daily fall rates between 10 and 15 mites per day. The mean fall rate for the 20 colonies was 34 mites per day and median 11 mites per day with interquartile range 5.1–38.9. Mite fall also varied considerably from day to day within colonies, particularly in the colonies with a lower fall rate (Figure 2).
Figure 2. Mite fall rate (mites per day) at each count over 30 days with 1 count approximately every 2 days for the Bee Gym treatment colonies (n = 10) and the control colonies (n = 10). Bee Gym or control frame treatment started on day 15. Counts where the fall rate was 0 are plotted as solid circles, and have been given a nominal value of 0.3 mites per day, to allow plotting on the log axis.
Proportional changes in mite fall
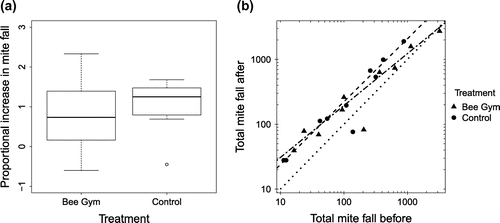
The majority of colonies showed an increase in mite fall from the first 14 days (fall before) to the second 14 days (fall after). The median proportional increase in fall from before to after was 0.73 for the Bee Gym colonies (interquartile range 0.22–1.24) and 1.25 (0.89–1.45) for the control colonies, and there was a larger variability in proportional increase for the Bee Gym colonies (Figure 3(a)). There was no significant difference in proportional increase in mite fall between the Bee Gym and control colonies (Two-sample Wilcoxon test, W = 35, p = 0.28).
Figure 3. The change in mite fall for the Bee Gym and control colonies represented as: (a) Proportional increase in mite fall from the first 14 days to the second 14 days for the Bee Gym (n = 10) and control colonies (n = 10), giving medians (central line), and interquartile ranges (boxes); whiskers include all observations within 1.5 interquartile ranges of the boxes, (b) Total mite fall after Bee Gym/control frame insertion vs. total mite fall before insertion for Bee Gym (triangles) (n = 10) and control (solid circles) colonies (n = 10). Mite fall was adjusted for the number of hours the drop boards were in place, giving non-integer values. Fitted regression lines are for the data with the two outliers removed (dashed line = control hives; dashed and dotted line = Bee Gym hives); the dotted line is y = x representing no difference from before to after.
Absolute changes in mite fall
Mite fall after was significantly correlated with fall before for the log-transformed data (slope = 0.848, CI 95% = [0.695; 1.001], intercept = 1.28, CI 95% = [0.49; 2.07], adjusted R2 = 0.88, F = 136, p < 0.0001) (Figure 3(b)). If the Bee Gym significantly increased mite fall for all colonies (regardless of the level of mite fall), this would be reflected in a significant increase in intercept of the regression model, assuming no significant change in slope. If the Bee Gym increased mite fall differently between colonies depending on their fall rate, this would be reflected in a significant change in the slope of the model. There were no significant differences between the Bee Gym and control colonies in slope (t = 1.04, p = 0.31) or intercept (t = 0.762, p = 0.46) of the regression fit.
Two colonies had a markedly smaller fall after than fall before (Figure 3(b)), one with Bee Gym treatment and one control colony, and these two colonies are notable outliers in the model fit with large residuals. As it is possible that a change in colony state could have caused the low fall after in these hives, the data were remodeled with these colonies removed (giving N = 18). For this new regression fit for the Bee Gym colonies there was a small significant decrease in the slope (β = −0.169, CI 95% [−0.294; −0.044], t = 2.90, p = 0.012), and marginally significant increase in intercept (β = 0.640, CI 95% [0.004; 1.276], t = 2.16, p = 0.049) compared to the regression fit for the control colonies (Figure 3(b)). Thus mite “fall after” in high-infestation colonies was slightly lower for the Bee Gym hives than for the control colonies.
Mite damage
Mites that fall from a hive as a result of grooming activities may be more likely to be damaged, thus mite fall was split into damaged and undamaged mites. There was no significant difference between the percentage of mites which were damaged for the Bee Gym and the control colonies (z = 0.272, p = 0.79) with respective model-predicted means 34.0% CI 95% [31.8; 36.2] and 34.3% CI 95% [32.7; 35.9].
Discussion
The effect of the Bee Gym on honey bee grooming of V. destructor was tested with two measures of mite fall out of a colony: total fall, and the proportion of damaged mites. Mite fall generally increased from the 14 days pre-treatment to the 14 days with Bee Gym or control treatment, as expected given typical mite population growth over the summer when brood are being raised (Boecking & Genersch, 2008Boecking, O., & Genersch, E. (2008). Varroosis—The ongoing crisis in bee keeping. Journal Fur Verbraucherschutz Und Lebensmittelsicherheit, 3, 221–228. doi:10.1007/s00003-008-0331-y[CrossRef], [Web of Science ®] [Google Scholar]). If bees were able to remove more mites through grooming in the hives with the Bee Gym fitted, we would expect to see an increase in the mite fall for the 14 days with treatment over that observed in the control colonies. However, there was no difference in the mite fall increase between the Bee Gym treatment and the control colonies. There was also no difference between the percentage of damaged mites between the Bee Gym and control colonies.
The significant difference of the slope of the regression line between treatment and control colonies when the data were remodeled with the two outliers removed implies that the Bee Gym colonies with high initial fall had a lower fall after, compared to the control hives of similar initial fall. This is contrary to the expected effect if the Bee Gym was effective as a grooming device. The coincident increase in intercept is small and only marginally significant and is not well reflected in the actual values for Bee Gym colonies with low fall (Figure 3(b)). Moreover, the significant difference in slope appears to be the result of a few high-leverage points, corresponding to high fall colonies, affecting the fit of the regression line (Figure 3(b)) and should be treated with caution. We conclude that this change in slope is not evidence of a significant positive effect of the Bee Gym. Hence, there is no clear evidence from this study that the Bee Gym increases mite fall through enhanced grooming by the bees.
The presence of brood has a large effect on mite fall (Branco et al., 2006Branco, M., Kidd, N., & Pickard, R.(2006). A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie, 37, 452–461. doi:10.1051/apido:2006010[CrossRef], [Web of Science ®] [Google Scholar]; Lobb & Martin, 1997Lobb, N., & Martin, S. (1997). Mortality of Varroa jacobsoni Oudemans during or soon after the emergence of worker and drone honey bees Apis mellifera L. Apidologie, 28, 367–374. doi:10.1051/apido:19970604[CrossRef], [Web of Science ®] [Google Scholar]). When brood is present, the majority of mites on drop boards are those associated with emerging brood (Lobb & Martin, 1997Lobb, N., & Martin, S. (1997). Mortality of Varroa jacobsoni Oudemans during or soon after the emergence of worker and drone honey bees Apis mellifera L. Apidologie, 28, 367–374. doi:10.1051/apido:19970604[CrossRef], [Web of Science ®] [Google Scholar]), with hygienic behavior and phoretic mites making up the remainder. As the Bee Gym is predicted to target phoretic mites, any effect would be to increase this portion of mite fall only. Many of the colonies had comparatively high V. destructor infestation, given their observed daily fall rates (Figure 2). If the Bee Gym only had a small effect on increasing mite fall through increased grooming, this may have been undetected because of the large numbers of mites associated with emerging brood in these high infestation colonies.
Mite fall was highly variable within colonies from count to count (Figure 2). Although it is possible that this variability could also obscure any increase in the portion of mite fall comprised of groomed phoretic mites, here mite fall was summed over two-week periods. Mite fall counted over two weeks has been shown to have a good correlation with colony mite population (Branco et al., 2006Branco, M., Kidd, N., & Pickard, R.(2006). A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie, 37, 452–461. doi:10.1051/apido:2006010[CrossRef], [Web of Science ®] [Google Scholar]) averaging out day-to-day variability. For the majority of the colonies, fall before showed a good correlation with fall after in the regression fit of log-log data (Adjusted R2 = 0.88, F = 136, p < 0.0001) (Figure 3(b)), suggesting that brood or other effects on mite fall were relatively constant when averaged over the recording period.
Recording the proportion of damaged mites may avoid the problem of the source of the fallen mites, by more directly considering that portion of mite fall related to grooming (Andino & Hunt, 2011Andino, G.K., & Hunt, G.J. (2011). A scientific note on a new assay to measure honey bee mite-grooming behavior. Apidologie, 42, 481–484. doi:10.1007/s13592-011-0004-1[CrossRef], [Web of Science ®] [Google Scholar]). The observed percentages of damaged mites recorded here, 34.0% for the Bee Gym and 34.3% for the control colonies, are similar to results from previous studies; for example Rinderer et al. (2013Rinderer, T.E., De Guzman, L.I., & Frake, A.M. (2013). Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology, 106, 566–575. doi:10.1603/EC12427[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]) recorded 29% mites as damaged. There was no significant difference observed between the Bee Gym and control colonies.
There is, however, some debate about the reliability of using damaged mites for determining grooming level of a colony (Guzmán-Novoa et al., 2012Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Rinderer et al., 2010Rinderer, T.E., Harris, J.W., Hunt, G.J., & de Guzman, L.I. (2010). Breeding for resistance to Varroa destructor in North America. Apidologie, 41, 409–424. doi:10.1051/apido/2010015[CrossRef], [Web of Science ®] [Google Scholar]). Damaged mites may result from causes other than grooming by adult bees (Rinderer et al., 2010Rinderer, T.E., Harris, J.W., Hunt, G.J., & de Guzman, L.I. (2010). Breeding for resistance to Varroa destructor in North America. Apidologie, 41, 409–424. doi:10.1051/apido/2010015[CrossRef], [Web of Science ®] [Google Scholar]). It is also not known whether mites removed through grooming using an external object would show similar damage to those removed by auto- and allo-grooming. It has been suggested that damage to mites from bees using their mandibles may mainly result from allo-grooming (Invernizzi, Zefferino, Santos, Sánchez, & Mendoza, 2015Invernizzi, C., Zefferino, I., Santos, E., Sánchez, L., & Mendoza, Y. (2015). Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. Journal of Apicultural Research, 54, 321–327. doi:10.1080/00218839.2016.1159055[Taylor & Francis Online], [Web of Science ®] [Google Scholar]). If this were the case here, then mite damage would not be a reliable indicator of efficacy of the Bee Gym. Different honey bee strains may differ in their ability to remove mites through grooming (Bahreini & Currie, 2015Bahreini, R., & Currie, R.W. (2015). The effect of queen pheromone status on Varroa mite removal from honey bee colonies with different grooming ability. Experimental and Applied Acarology, 66, 383–397. doi:10.1007/s10493-015-9907-2[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]; Guzmán-Novoa et al., 2012Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]). Here, the genetic background of the colonies was not controlled, and a grooming-dependent treatment might only be effective in high-grooming honey bee strains.
Although the drop boards were covered with petroleum jelly to both trap the mites and prevent removal of trapped mites by other insects, ants were observed on the drop boards of some hives. It is possible that these affected the counts of mite fall (Dainat, Kuhn, Cherix, & Neumann, 2011Dainat, B., Kuhn, R., Cherix, D., & Neumann, P. (2011). A scientific note on the ant pitfall for quantitative diagnosis of Varroa destructor. Apidologie, 42, 740–742. doi:10.1007/s13592-011-0071-3[CrossRef], [Web of Science ®] [Google Scholar]). Petroleum jelly is suggested as a preventative measure to stop ant predation of V. destructor from drop boards (Dietemann et al., 2013Dietemann, V., Nazzi, F., & Martin, S.(2013). Standard methods for varroa research. In V. Dietemann, J.D. Ellis, & P. Neumann (Eds.), The COLOSS BEEBOOK, Volume II: Standard methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research, 52. doi:10.3896/IBRA.1.52.1.09[Taylor & Francis Online] [Google Scholar]) but we suggest that more substantial measures, such as those used by Dainat et al. (2011Dainat, B., Kuhn, R., Cherix, D., & Neumann, P. (2011). A scientific note on the ant pitfall for quantitative diagnosis of Varroa destructor. Apidologie, 42, 740–742. doi:10.1007/s13592-011-0071-3[CrossRef], [Web of Science ®] [Google Scholar]), should be taken to prevent ant predation when measuring V. destructor fall.
Grooming is a promising area for improving V. destructor management (Rinderer et al., 2013Rinderer, T.E., De Guzman, L.I., & Frake, A.M. (2013). Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology, 106, 566–575. doi:10.1603/EC12427[CrossRef], [PubMed], [Web of Science ®] [Google Scholar]); however, from this investigation we find no detectable effect of the Bee Gym grooming device on V. destructor removal in honey bee colonies over a 2 week period. The measures used for assessing grooming efficacy are still under debate, and it is possible that an effect could be missed, or obscured by other confounding factors. Nevertheless, to our knowledge, there is currently no evidence that bees use external objects to groom. If further work is carried out on this as a potential treatment method, we therefore suggest that a sensible initial step would be to directly test the potential of grooming devices in assays with individual bees.
Disclosure statement
In accordance with Taylor & Francis policy and our ethical obligations as researchers, we are reporting that we received funding (as detailed below) from Stuart Roweth, the inventor of the Bee Gym, who may be affected by the research reported in the enclosed paper. We have disclosed these interests fully to Taylor & Francis.
Funding
Stuart Roweth supplied the Bee Gyms and control frames and provided funding for equipment and travel costs. JGP was funded by a Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentship under Grant [BB/J014540/1].
Underlying research materials
The underlying research materials for this article can be accessed at http://dx.doi.org/10.17863/CAM.6364.
Acknowledgements
We gratefully acknowledge the beekeepers managing the hives in this experiment: Helen Andre-Cripps, Rosie Bolton, Maddie Geddes-Barton, Tony Harte, and Richard Steel. We thank the Cambridgeshire Beekeepers’ Association for access to their apiary at Impington, and Trinity College and King’s College, Cambridge, and the Cambridge University Botanic Garden for facilitating access to their hives. We also thank David Labonte for helpful discussions on an earlier version of this manuscript.
References
- Andino, G.K., & Hunt, G.J. (2011). A scientific note on a new assay to measure honey bee mite-grooming behavior. Apidologie, 42, 481–484. doi:10.1007/s13592-011-0004-1[CrossRef], [Web of Science ®]
[Google Scholar] - Arechavaleta-Velasco, M., & Guzmán-Novoa, E. (2001). Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie, 32, 157–174. doi:10.1051/apido:2001121[CrossRef], [Web of Science ®]
[Google Scholar] - Aumeier, P. (2001). Bioassay for grooming effectiveness towards Varroa destructor mites in Africanized and Carniolan honey bees. Apidologie, 32, 81–90. doi:10.1051/apido:2001113[CrossRef], [Web of Science ®]
[Google Scholar] - Bahreini, R., & Currie, R.W. (2015). The effect of queen pheromone status on Varroa mite removal from honey bee colonies with different grooming ability. Experimental and Applied Acarology, 66, 383–397. doi:10.1007/s10493-015-9907-2[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Boecking, O., & Genersch, E. (2008). Varroosis—The ongoing crisis in bee keeping. Journal Fur Verbraucherschutz Und Lebensmittelsicherheit, 3, 221–228. doi:10.1007/s00003-008-0331-y[CrossRef], [Web of Science ®]
[Google Scholar] - Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30, 141–158. doi:10.1051/apido:19990205[CrossRef], [Web of Science ®]
[Google Scholar] - Bowen-Walker, P.L., & Gunn, A. (2001). The effect of the ectoparasitic mite, Varroa destructor on adult worker honey bee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomologia Experimentalis et Applicata, 101, 207–217. doi:10.1046/j.1570-7458.2001.00905.x[CrossRef], [Web of Science ®]
[Google Scholar] - Bowen-Walker, P.L., Martin, S.J., & Gunn, A. (1999). The transmission of deformed wing virus between honey bees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. Journal of Invertebrate Pathology, 73, 101–106. doi:10.1006/jipa.1998.4807[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Branco, M., Kidd, N., & Pickard, R. (2006). A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie, 37, 452–461. doi:10.1051/apido:2006010[CrossRef], [Web of Science ®]
[Google Scholar] - Büchler, R., Drescher, W., & Tornier, I. (1992). Grooming behaviour of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Experimental and Applied Acarology, 16, 313–319. doi:10.1007/BF01218573[CrossRef], [Web of Science ®]
[Google Scholar] - Calderone, N. (2005). Evaluation of drone brood removal for management of Varroa destructor (Acari: Varroidae) in colonies of Apis mellifera (Hymenoptera: Apidae) in the northeastern United States. Journal of Economic Entomology, 98, 645–650. doi:10.1603/0022-0493-98.3.645[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Dainat, B., Kuhn, R., Cherix, D., & Neumann, P. (2011). A scientific note on the ant pitfall for quantitative diagnosis of Varroa destructor. Apidologie, 42, 740–742. doi:10.1007/s13592-011-0071-3[CrossRef], [Web of Science ®]
[Google Scholar] - Davis, A. (2009). Regular dorsal dimples on Varroa destructor—Damage symptoms or developmental origin. Apidologie, 40, 151–162. doi:10.1051/apido/2009001[CrossRef], [Web of Science ®]
[Google Scholar] - Dietemann, V., Nazzi, F., & Martin, S. (2013). Standard methods for varroa research. In V. Dietemann, J.D. Ellis, & P. Neumann (Eds.), The COLOSS BEEBOOK, Volume II: Standard methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research, 52. doi:10.3896/IBRA.1.52.1.09[Taylor & Francis Online]
[Google Scholar] - Evans, J., & Spivak, M. (2010). Socialized medicine: Individual and communal disease barriers in honey bees. Journal of Invertebrate Pathology, 103, S62–72. doi:10.1016/j.jip.2009.06.019[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Fries, I., Huazhen, W., Wei, S., & Jin, C.S. (1996). Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie, 27, 3–11. doi:10.1051/apido:19960101[CrossRef], [Web of Science ®]
[Google Scholar] - Guzmán-Novoa, E., Eccles, L., Calvete, Y., McGowan, J., Kelly, P., & Correa-Benítez, A. (2010). Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie, 41, 443–450. doi:10.1051/apido/2009076[CrossRef], [Web of Science ®]
[Google Scholar] - Guzmán-Novoa, E., Emsen, B., Unger, P., Espinosa-Montaño, L.G., & Petukhova, T. (2012). Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). Journal of Invertebrate Pathology, 110, 314–320. doi:10.1016/j.jip.2012.03.020[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Harris, J.W. (2007). Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged ≤ five days post capping. Journal of Apicultural Research, 46, 134–139. doi:10.3896/IBRA.1.46.3.02[Taylor & Francis Online], [Web of Science ®]
[Google Scholar] - Higes, M., Meana, A., Suárez, M., & Llorente, J. (1999). Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoni Oud. Apidologie, 30, 289–292. doi:10.1051/apido:19990404[CrossRef], [Web of Science ®]
[Google Scholar] - Invernizzi, C., Zefferino, I., Santos, E., Sánchez, L., & Mendoza, Y. (2015). Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. Journal of Apicultural Research, 54, 321–327. doi:10.1080/00218839.2016.1159055[Taylor & Francis Online], [Web of Science ®]
[Google Scholar] - Land, B.B., & Seeley, T.D. (2004). The grooming invitation dance of the honey bee. Ethology, 110, 1–10. doi:10.1046/j.1439-0310.2003.00947.x[CrossRef], [Web of Science ®]
[Google Scholar] - Le Conte, Y., Ellis, M., & Ritter, W. (2010). Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie, 41, 353–363. doi:10.1051/apido/2010017[CrossRef], [Web of Science ®]
[Google Scholar] - Lobb, N., & Martin, S. (1997). Mortality of Varroa jacobsoni Oudemans during or soon after the emergence of worker and drone honey bees Apis mellifera L. Apidologie, 28, 367–374. doi:10.1051/apido:19970604[CrossRef], [Web of Science ®]
[Google Scholar] - Lodesani, M., Colombo, M., & Spreafico, M. (1995). Ineffectiveness of Apistan® treatment against the mite Varroa jacobsoni Oud in several districts of Lombardy (Italy). Apidologie, 26, 67–72. doi:10.1051/apido:19950109[CrossRef], [Web of Science ®]
[Google Scholar] - Martin, S.J. (2004). Acaricide (pyrethroid) resistance in Varroa destructor. Bee World, 85, 67–69. doi:10.1080/0005772X.2004.11099632[Taylor & Francis Online], [Web of Science ®]
[Google Scholar] - Martin, S.J., & Medina, L.M. (2004). Africanized honey bees have unique tolerance to Varroa mites. Trends in Parasitology, 20, 112–114. doi:10.1016/j.pt.2004.01.001[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Mondragón, L., Spivak, M., & Vandame, R. (2005). A multifactorial study of the resistance of honey bees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie, 36, 345–358. doi:10.1051/apido:2005022[CrossRef], [Web of Science ®]
[Google Scholar] - Peng, Y., Fang, Y., Xu, S., & Ge, L. (1987). The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. Journal of Invertebrate Pathology,49, 54–60. doi:10.1016/0022-2011(87)90125-X[CrossRef], [Web of Science ®]
[Google Scholar] - Potts, S.G., Roberts, S.P.M., Dean, R., Marris, G., Brown, M., Jones, R., & Settele, J. (2010). Declines of managed honey bees and beekeepers in Europe. Journal of Apicultural Research, 49, 15–22. doi:10.3896/IBRA.1.49.1.02[Taylor & Francis Online], [Web of Science ®]
[Google Scholar] - Pritchard, D.J. (2016). Grooming by honey bees as a component of varroa resistant behavior. Journal of Apicultural Research, 55, 38–48. doi:10.1080/00218839.2016.1196016[Taylor & Francis Online], [Web of Science ®]
[Google Scholar] - R Core Team. (2015). R: A language and environment for statistical computing (Version 3.1.3). Vienna, Austria: R Foundation for Statistical Computing.
[Google Scholar] - Rademacher, E., & Harz, M. (2006). Oxalic acid for the control of varroosis in honey bee colonies—A review. Apidologie, 37, 98–120. doi:10.1051/apido:2005063[CrossRef], [Web of Science ®]
[Google Scholar] - Rinderer, T.E., De Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V., … Harris, J.W.(2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie, 32, 381–394. doi:10.1051/apido:2001138[CrossRef], [Web of Science ®]
[Google Scholar] - Rinderer, T.E., De Guzman, L.I., & Frake, A.M. (2013). Associations of parameters related to the fall of Varroa destructor (Mesostigmata: Varroidae) in Russian and Italian honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology, 106, 566–575. doi:10.1603/EC12427[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar] - Rinderer, T.E., Harris, J.W., Hunt, G.J., & de Guzman, L.I. (2010). Breeding for resistance to Varroa destructor in North America. Apidologie, 41, 409–424. doi:10.1051/apido/2010015[CrossRef], [Web of Science ®]
[Google Scholar] - Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119. doi:10.1016/j.jip.2009.07.016[CrossRef], [PubMed], [Web of Science ®]
[Google Scholar]